- DEBRA “The Butterfly Children Charity”continues to work so that the “timeline” established by law to include a new wound healing treatment on the National Health Service, be met as quickly as possible.
- Families with Butterfly Skin will have to wait approximately 3 years until this treatment is made available. Legislation states that this should take a maximum of 6 months.
- Historically Spain is one of the EC countries that takes the most time to offer treatments for rare diseases on the National Health Service.
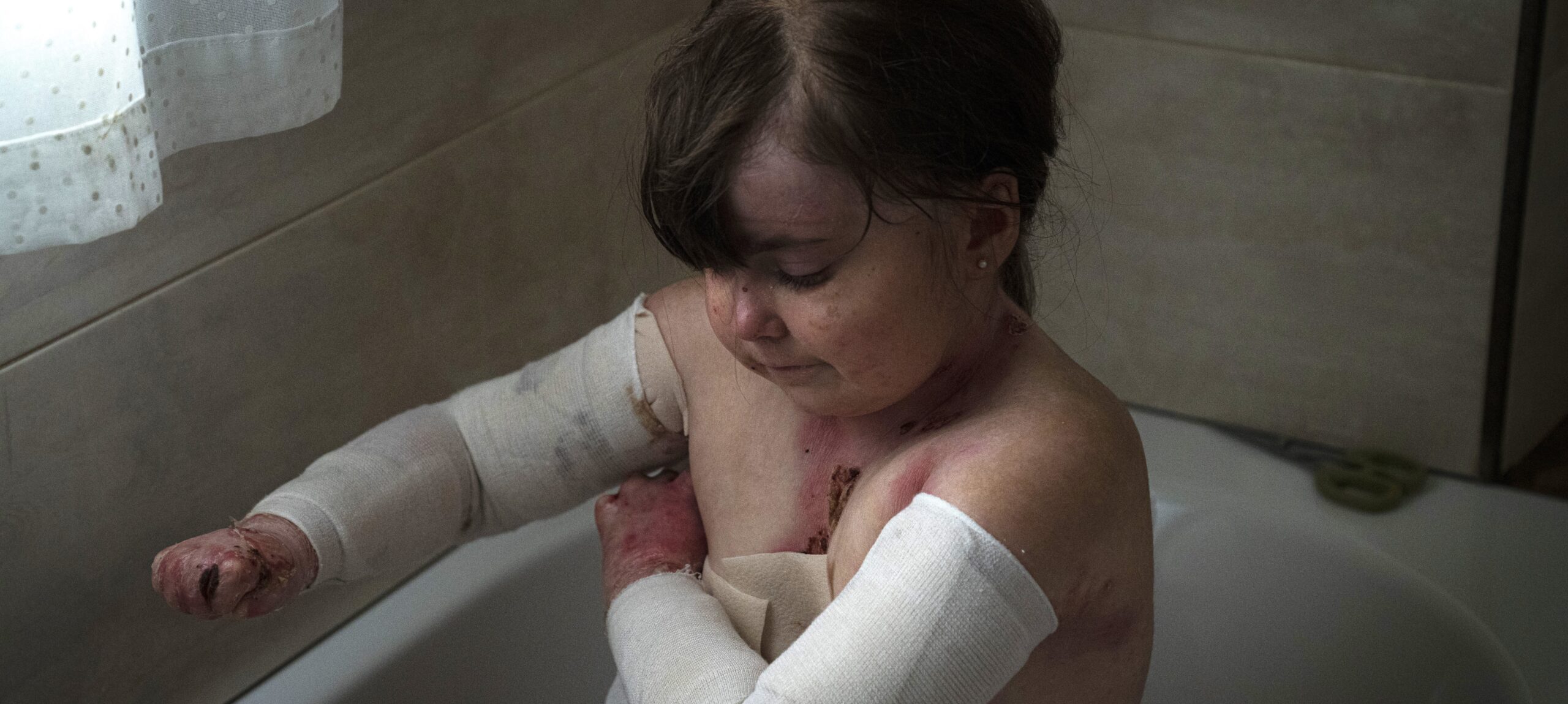
- Butterfly Skin is a rare, degenerative and currently incurable disease that causes painful open wounds, covering 90% of the surface of the skin. DEBRA the Butterfly Children Charity is the only organisation in Spain dedicated to improving the quality of life for families.
When a groundbreaking new treatment, FILSUVEZ, made by Amryt Pharma (now Chiesi), was approved by the European Medicines Agency in June 2022, families with Epidermolysis bullosa or Butterfly Skin, were finally given some hope.
“Access to this topical treatment for Dystrophic and Junctional EB could lead to a substantial improvement in the quality of life for families living with this debilitating and painful condition. Patients endure up to 5 hours a day of painful wound care management and treatment. This medication could make a significant difference to families” said Evanina Morcillo Makow, manager of the charity. However, the approval for this treatment, which accelerates the healing process, has been delayed. Families need to access this treatment as soon as possible to avoid further suffering. They have already been waiting for 2 years and will need to wait a further 3 years for the treatment to be offered by the National Health Service. In this time wounds will continue to appear and the complications, including severe infections and carcinomas will continue to develop.
“The process is so frustrating for families, and we will continue to push the health authorities to expedite this process so that families can start to benefit from this new treatment.”
Requests for treatment or medication from other countries
Filsuvez is already available on the national health service in other countries like Germany. Some families have been able to access the medication through their hospital as a request for medication under special circumstances. The problem, according to DEBRA, is that families find themselves battling with further bureaucratic complications and a tiring and tedious administrative process, which often ends in further despair as the medication is unauthorised. According to Nuria Tarrats, DEBRA researcher, this happens for a number of different reasons:
“The inequality stems from the fact that Spain has 17 different health systems. Depending on where the families live, they will have more or less access to treatment and different medications, this is due to the way each hospital works and the protocol of individual health authorities, but it also depends on the knowledge of the health team and how up to date they are on new treatments”.


Rare diseases are often left untreated
It is important to underline that Spain is one of the European Union countries with the least available approved treatments available on the National Health Service (78 in contrast to 147 in the rest of Europe). There are currently only 51% of orphan drugs (drugs that treat rare diseases) available that could help families with EB, in contrast with 80% in Italy and 90% in Germany.
There are currently 45 orphan drugs waiting for funding in Spain. Almost half have been waiting for 3 years to become available on the National Health Service, the main reason is due to the lack of state funding as they evaluate both the public expenditure and the therapeutic and social value of a new treatment. As this is for a rare disease and will only treat a small percentage of the population, it is not considered to be a priority despite the severity of living with a rare condition like EB.
Other treatments giving hope for Butterfly Skin families
VYJUVEK, a treatment promoted by Krystal Biotech and developed by the university of Standford, is one of the most promising treatments developed to date for the treatment of dystrophic EB. It is a genetic, topical medication which provides the information needed for the cells to produce collagen 7 (the protein responsible for gluing the dermis and the epidermis together, and that also makes the skin resistant).
We are hopeful that it will be approved by the European Commission by October of 2024.